
HL Paper 1
The graph shows the first ionization energies of some consecutive elements.
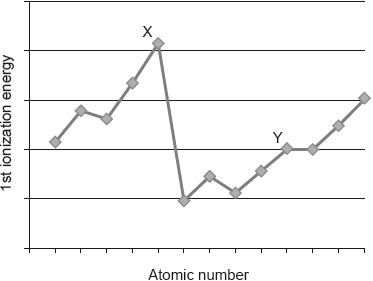
Which statement is correct?
A. Y is in group 3
B. Y is in group 10
C. X is in group 5
D. X is in group 18
Markscheme
D
Examiners report
Values for the successive ionization energies for an unknown element are given in the table below.

In which group of the periodic table would the unknown element be found?
A. 1
B. 2
C. 3
D. 4
Markscheme
A
Examiners report
Between which ionization energies of boron will there be the greatest difference?
A. Between 1st and 2nd ionization energies
B. Between 2nd and 3rd ionization energies
C. Between 3rd and 4th ionization energies
D. Between 4th and 5th ionization energies
Markscheme
C
Examiners report
What is the electron configuration of the copper(I) ion, \({\text{C}}{{\text{u}}^ + }\)?
A. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{1}}}{\text{3}}{{\text{d}}^{\text{9}}}\)
B. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{d}}^{\text{8}}}\)
C. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{1}}}{\text{3}}{{\text{d}}^{{\text{10}}}}\)
D. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{{\text{10}}}}\)
Markscheme
D
Examiners report
The first ionization energies (in \({\text{kJmo}}{{\text{l}}^{ - 1}}\)) of five successive elements in the periodic table are:
1314, 1681, 2081, 496 and 738
What could these elements be?
A. d-block elements
B. The last two elements of one period and the first three elements of the next period
C. The last three elements of one period and the first two elements of the next period
D. The last five elements of a period
Markscheme
C
Examiners report
Which equation represents the second ionization energy of potassium?
A. \({\text{K(g)}} \to {{\text{K}}^{2 + }}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }\)
B. \({{\text{K}}^ + }{\text{(g)}} \to {{\text{K}}^{2 + }}{\text{(g)}} + {{\text{e}}^ - }\)
C. \({\text{K(s)}} \to {{\text{K}}^{2 + }}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }\)
D. \({{\text{K}}^ + }{\text{(s)}} \to {{\text{K}}^{2 + }}{\text{(g)}} + {{\text{e}}^ - }\)
Markscheme
B
Examiners report
Successive ionization energies for an element, Z, are shown in the table below.
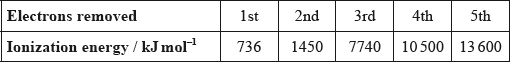
What is the most likely formula for the ion of Z?
A. \({{\text{Z}}^ + }\)
B. \({{\text{Z}}^{2 + }}\)
C. \({{\text{Z}}^{3 + }}\)
D. \({{\text{Z}}^{4 + }}\)
Markscheme
B
Examiners report
The graph below shows the first four ionization energies of four elements A, B, C and D (the letters are not their chemical symbols). Which element is magnesium?
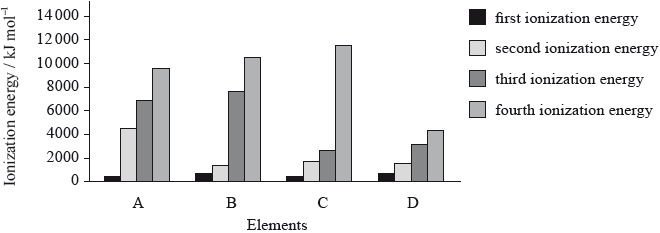
Markscheme
B
Examiners report
Which transition on the diagram corresponds to the ionization of hydrogen in the ground state?
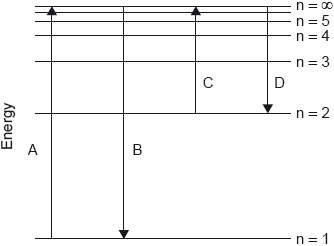
Markscheme
A
Examiners report
A period 3 element, M, forms an oxide of the type M2O. Which represents the first four successive ionization energies of M?
Markscheme
A
Examiners report
The diagram shows the first ionization energies of four consecutive elements in the periodic table. Which element is in Group 14?
Markscheme
B
Examiners report
Which statement explains one of the decreases in first ionization energy (I.E.) across period 3?
A. The nuclear charge of element Al is greater than element Mg.
B. The electron-electron repulsion is greater, for the electron with the opposite spin, in element S than in element P.
C. A new sub-level is being filled at element S.
D. The p orbital being filled in element Al is at a lower energy than the s orbital in element Mg.
Markscheme
B
Examiners report
The graph represents the first ten ionisation energies (IE) of an element.
What is the element?
A. O
B. S
C. Ne
D. Cl
Markscheme
B